There are 118 different elements on the periodic table, and each one is unique depending on its chemical properties. Elements are categorized into three main groups: metals, nonmetals, and metalloids. Let’s discuss each classification, their properties, and some real-world examples.
What Is a Metal?

Metals are periodic elements characterized by similar traits and molecular structures. They are typically hard, shiny materials with good thermal and electrical conductivity. Metals are also known for their unique malleable, ductile, and reflective properties that make them suitable for construction, manufacturing, and medical applications.
There are over 90 types of metal on the periodic table, which are classified differently depending on physical or chemical properties. In industrial applications, iron content is used to classify workpiece metals into three categories:
- Ferrous (contains iron)
- Nonferrous (contains no iron)
- Alloys (contains multiple metals and other elements)
Properties of Metals
A metal’s unique properties differentiate it from other elements. Below are some notable properties of metals:
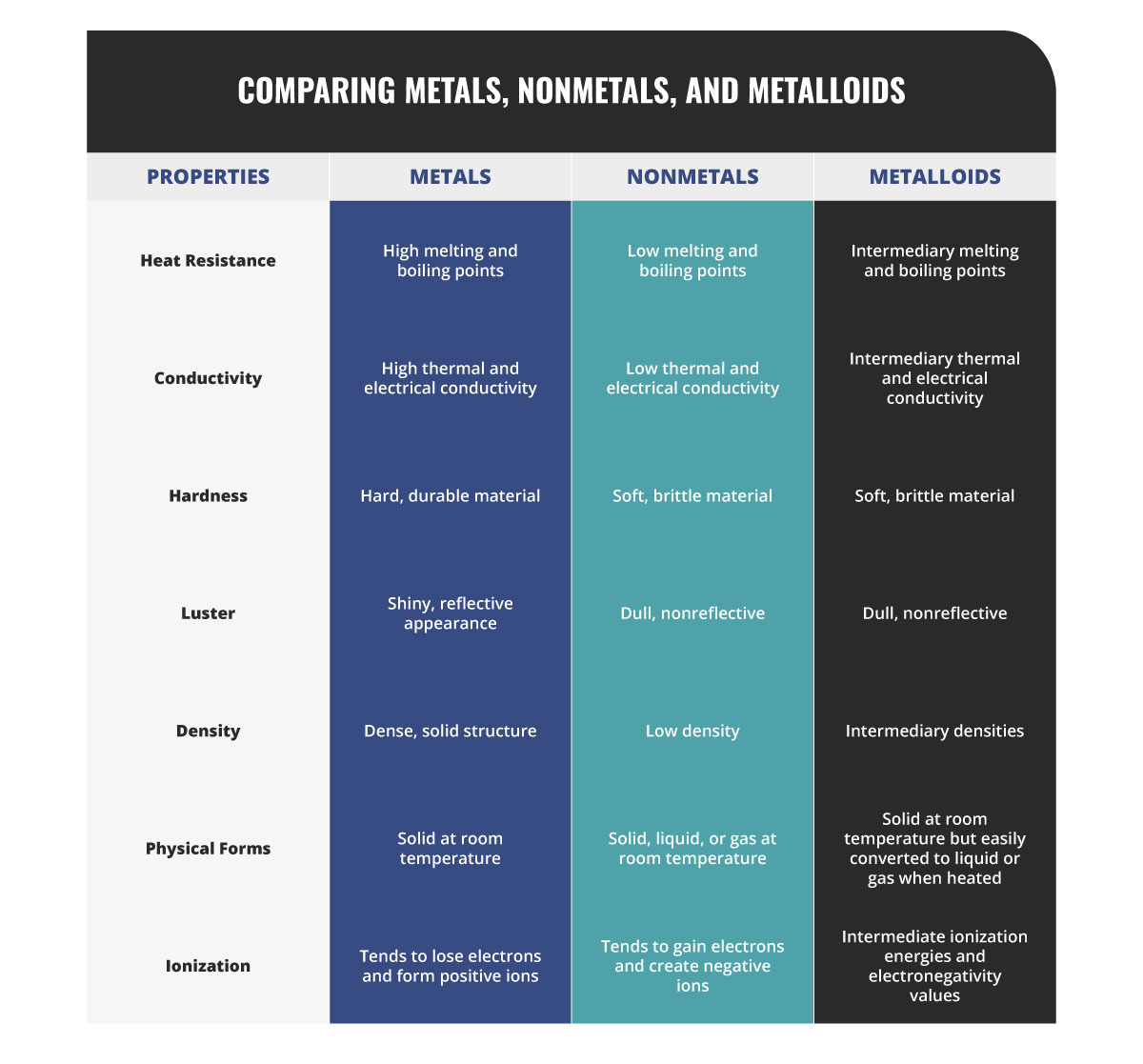
- Heat resistance: Metals have high melting and boiling points, allowing them to remain solid in high temperatures.
- Conductivity: Metals are known for their ability to conduct electricity and heat due to free electrons that move freely throughout the metal and carry electrical and thermal current.
- Malleability and ductility: Metals are malleable and ductile, allowing them to be pounded, rolled into different shapes, or drawn into wire without breaking.
- Luster: Most metals have a shiny or metallic appearance known as luster, which reflects light.
- Density: Metals are dense, solid materials that weigh more than nonmetals.
- Physical forms: Most metals are naturally solid at room temperature, except for mercury, which is liquid at room temperature.
- Form alloys: Metals can be mixed with other elements to form alloys, which are materials with improved properties (such as increased strength or corrosion resistance).
- Ionization: Metals are prone to losing electrons and forming positive ions. This is because the outermost electrons in a metal’s atoms are not strongly attracted to the positively charged nucleus and can be easily removed.
Examples of Metals
Here are the three most common examples of industrial metals and their alloys:
Iron
Iron is the most abundant metal on earth by volume, making up approximately 5% of the earth’s crust. Similarly, 90% of all manufactured metals are iron-based, including steel. Pure iron is widely used in cookware, heat-producing appliances (like stoves), and heavy machinery. High melting points and strong rigidity provide stability and safety in high-temperature environments. The two primary types of iron alloys are wrought iron and cast iron.
Steel
Steel is a ferrous metal used for projects of all sizes, ranging from skyscrapers to surgical instruments. It’s relatively low-cost to manufacture, which makes it ideal for mass production. The three primary steel alloys are carbon steel, alloy steel, and stainless steel.
Copper
Copper is a nonferrous metal used in industrial manufacturing for over 6,000 years. It is a high electrical and thermal conductor in addition to being corrosion-resistant. Copper is used in electronics, piping, and telecommunications around the world. Its noncorrosive properties make it long-lasting and low-maintenance. The two primary types of copper alloys are brass and bronze.
What Is a Nonmetal?

As the name suggests, nonmetals are elements characterized by their lack of metallic properties. They are often brittle and poor conductors of heat and electricity. Nonmetals are natural elements used in pure form and in the form of compounds when combined with other elements.
There are 20 nonmetals on the periodic table, which are categorized into three subgroups:
- Nonmetals: Elements that are most commonly thought of as nonmetals. They include elements such as sulfur, phosphorus, chlorine, and carbon.
- Halogens: Elements with properties of both metals and nonmetals. They include elements such as silicon, boron, and arsenic.
- Noble gasses: Elements that are highly stable and unreactive such as helium, neon, and argon. Noble gasses are often considered a separate group from nonmetals, although they are technically nonmetals.
Properties of Nonmetals
Below are some notable properties of nonmetals:
- Heat resistance: Nonmetals have low melting and boiling points and require relatively little heat to convert from a solid to a liquid or a gas.
- Conductivity: Nonmetals are poor conductors of electricity and heat.
- Hardness: Nonmetals tend to be brittle and break or shatter easily when stressed.
- Luster: Nonmetals are generally non-reflective, dull, and lack a shiny or metallic surface.
- Density: Nonmetals are generally less dense than metals.
- Physical forms: Nonmetals exist in solid, liquid, or gas forms at room temperature.
- Ionization: Nonmetals tend to gain electrons and create negative ions or anions.
Examples of Nonmetals
Here are some common examples of nonmetals and their applications:
- Carbon: Carbon is an essential component of steel and other alloys and is also used to make a variety of polymers such as plastics, resins, and rubber.
- Chlorine: Chlorine is used to purify water and make a variety of chemicals such as bleach, plastics, and pharmaceuticals.
- Fluorine: Fluorine is used to make a variety of chemicals such as refrigerants, solvents, and pharmaceuticals. It is also added to water supplies to help prevent tooth decay.
- Hydrogen: Hydrogen is a fuel source in addition to creating a variety of chemicals such as ammonia and methanol.
- Nitrogen: Nitrogen is used to create fertilizers and a variety of chemicals such as explosives and dyes.
- Oxygen: Oxygen is necessary for life and is also used in various industrial processes such as welding and metal cutting.
- Phosphorus: Phosphorus is an essential element for life and is used to make a variety of chemicals such as detergents, insecticides, and flame retardants.
- Sulfur: Sulfur is used to create fertilizers and sulfuric acid — a crucial part of many industrial processes.
What Is a Metalloid?
Metalloids are the middle ground between metals and nonmetals, exhibiting qualities of both groups of elements. They are more thermally or electrically conductive than nonmetals, but not as much as metals. Metalloids are metallic in appearance but are brittle and extremely fragile despite being solid at room temperature.
Properties of Metalloids
Metalloids share many similar properties with metals and nonmetals. Their properties are an intermediary mix between the two, determined by an individual metalloid’s physical and chemical traits. For example, a metalloid can look like a metal physically but behave like a nonmetal chemically. Metalloids are valuable elements often used to create popular alloys and chemical compounds.
- Heat resistance: Metalloids have intermediate melting and boiling points and require more heat than nonmetals but less heat than metals to convert from a solid to a liquid or a gas.
- Conductivity: Metalloids are conductors of electricity and heat but are not as conductive as metals.
- Hardness: Metalloids tend to be brittle and break or shatter like nonmetals.
- Luster: Metalloids are generally reflective and shiny like metals.
- Density: Metalloids are generally less dense than metals but denser than nonmetals.
- Physical forms: Most metalloids are solid at room temperature but are converted to a liquid or gas when heated.
- Ionization: Metalloids have intermediate ionization energies and electronegativity values.
Examples of Metalloids
Below are seven commonly recognized metalloids used to create various alloys and chemical compounds. Metalloids are found in everything from food and dietary supplements to industrial metals and semiconductors.
- Boron
- Silicon
- Germanium
- Arsenic
- Antimony
- Tellurium
- Polonium
Understanding the Difference Between Metals, Nonmetals, and Metalloids
Metals are the largest group of elements on the periodic table, setting the standard for comparison across the three groups. Nonmetal and metalloid properties are based on where they stack up against metals. Let’s take a look at the differences between metals, nonmetals, and metalloids based on the following physical properties:
Appearance
- Metals: Most metals have a shiny metallic luster and reflective surface.
- Nonmetals: Nonmetals are generally dull or nonreflective and do not have a metallic luster.
- Metalloids: Metalloids can have a metallic or nonmetallic appearance, depending on the element.
Malleability
- Metals: Most metals are malleable and can be pounded or rolled into different shapes without breaking. The atoms in a metal are arranged in a regular, repeating pattern that allows them to slide past one another easily.
- Nonmetals: Nonmetals are generally not very malleable and will break when pounded or rolled. The atoms in a nonmetal are not arranged in a regular pattern and do not slide past one another easily.
- Metalloids: Some metalloids are malleable, while others are not. For example, silicon is a metalloid that is not very malleable, while boron is a metalloid that is quite malleable.
Ductility
- Metals: Metals are highly ductile and can be drawn into wire without breaking.
- Nonmetals: Nonmetals are not very ductile and cannot be drawn into wire without breaking.
- Metalloids: Some metalloids are ductile, while others are not.
Metal, Nonmetal, and Metalloid Comparison Chart
Want to Learn More About Different Metal Types?
At Mead Metals, we're experts in all things metal material. Our comprehensive "Complete Guide to Metal Products" covers:
- Advantages of different metal types for various applications
- Tips on choosing the right metal for your project needs
- Industry terms you need to know
- And much more







